
Sherita Holmes, MD sherita.holmes@emory.edu
Every year we expect a flu epidemic which usually starts in the fall and lasts until spring (as early as October and last until late May). According to the most recent CDC Weekly U.S. Influenza Surveillance Report (week 8 – February 25th), flu activity remains elevated in the United States; although it appears to be downtrending.1
We know that in a majority of patients; the flu manifests as a nuisance that causes our patients to have high fevers with self-limited respiratory symptoms, fatigue, and myalgias. However, we also know that in the very young (age < 2 years) or very old (age > 65 years) as well as those with underlying medical conditions (i.e. asthma, immunosuppression, diabetes, heart disease) [see Table 1]; the flu can be fatal. This flu season there have been 40 pediatric deaths reported thus far [Figure 1].1 This underscores the importance of prevention and why it is critical that we encourage flu vaccination, especially in these high risk groups.
While vaccination is important in preventing influenza, we can use antiviral medications to shorten the length of illness (by 1-2 days), reduce complications such as pneumonia, and lessen severity.2 Antiviral medications should be started as soon as possible – ideally within the first 48 hours of illness – for any patient with suspected or confirmed influenza who: has severe, complicated, or progressive illness; is in a high risk group; or is hospitalized.3 Consider chemoprophylaxis in patients in high risk groups with known exposure to influenza.
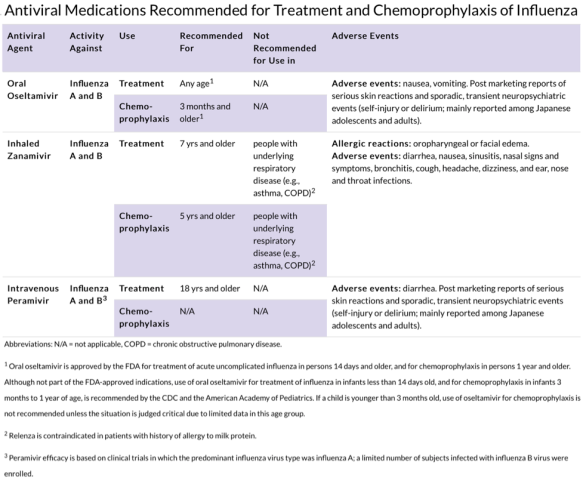
There are two classes of antivirals for influenza: neuraminidase inhibitors (oseltamivir, zanamivir) and adamantanes (amantadine, rimantadine). The adamantanes are not effective against influenza B and there are high levels of resistance against the current influenza A viruses. For 2016-2017 flu season, the CDC only recommends using the neuraminidase class of antivirals. Oseltamivir (Tamiflu) is available in pill or liquid form, while zanamivir (Relenza) only available in inhaled form. Please refer to Tables 2 and 3 for further information regarding antiviral medication age designations, contraindications, adverse effects, and dosages.3,4
In the battle against influenza, we must do our best to not only identify the appropriate patients that would benefit from antiviral medications, but most importantly to encourage all patients and their loved ones to get vaccinated.

Figure 1 CDC https://www.cdc.gov/flu/weekly/index.htm#S3

Table 3 Harper et al. https://doi.org/10.1086/598513
References:
- CDC Weekly U.S. Influenza Surveillance Report
https://gis.cdc.gov/grasp/fluview/flu_by_age_virus.html
- Campbell, Angela. CDC Expert Commentary 2016-2017 Influenza Antiviral Recommendations
http://www.medscape.com/partners/cdc/public/cdc-commentary
- Centers for Disease Control (CDC) and Prevention 2016-2017 Flu Season
https://www.cdc.gov/flu/about/season/current.htm
- Harper, S.A., Bradley, J.S., Englund, J.A., et al. Seasonal Influenza in Adults and Children—Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management: Clinical Practice Guidelines of the Infectious Diseases Society of America


You must be logged in to post a comment.